Source attribution can be made easier with the LI-7825 CO2 Isotope/NH3 Trace Gas Analyzer
Dave Lowry, a stable-isotope geochemist at Royal Holloway University of London (RHUL) in England, started his career studying the mineralization of deposits in the UK. His early work mostly involved the breakdown of carbonates by phosphoric acid.
In the late 1990s, he started work in the atmospheric sciences to assist with EU efforts to characterize methane emissions. At the time, measurements were made using a mass spectrometer. More recently, he has worked to develop techniques to expand the measurements to include δ13C. Dr. Lowry and his colleagues have collected many CO2 records over the years, but the records are not as informative as they could be because researchers do not yet understand the sources and source signatures of these emissions.
Source signatures – a measurable indicator of the source of a gas plume – can be made by isotopic analysis. This presents clear research opportunities, which can be filled by the LI-7825 CO2 Isotope/NH3 Trace Gas Analyzer.
Dr. Lowry tested a prototype of the LI-7825 to address these questions. Here we describe his tests, the results, and the new research opportunities that are made available by widespread δ13C measurements.
The Old Oak Tree
When air sampling lines were first installed for offices of Royal Holloway University of London, the old oak tree nearby was of little concern. At the time, the researchers were interested solely in methane. Carbon dioxide gas was not a concern. Later, CO2 gas analyzers were connected to the same sampling line, and that is when things got interesting. One warm spring day, when the oak tree buds burst open, they observed a sudden, dramatic decrease in the CO2 concentrations.
Despite the unexpected magnitude of the concentration changes, the measurements were not in vain. The observations led to an experiment in which researchers attempted to measure the effects of the tree on the isotopic signature. Diurnal cycles of the CO2 concentrations were evident. He also observed a strong effect of photosynthetic CO2 uptake and a negative shift in δ13C, indicating that the 13C is being depleted in the sampled air.
The Forest
Testing the LI-7825 at a forest research site, Dr. Lowry assessed data from an overnight period to identify CO2 source signatures. He observed significant differences between the evening peak before sunset and the wider, but smaller overnight peak. Optimizing the averaging period to 10 seconds brought out the most interesting features of the data, revealing the evening peak of δ13C of -31‰ and the overnight peak of -19‰. Although the reason for this difference, and thus the source, are not yet clear, vehicle exhaust is the most likely culprit. The location is relatively remote for the south of England, but there may be an influence of the evening rush hour – and this may be the subject of upcoming research.
The Mobile Survey
Deploying the instruments on their mobile platform in northwest England, the team surveyed a site that has known sources of CO2 and CH4 from nearby dairy farms and landfills.
The platform, which measured a single air stream, featured both the LI-7825 for CO2 and δ13C with the LI-7810 for methane. When measuring a plume downwind of the landfill site, the team could identify clear source signatures in the CO2 isotope measurements.
The mobile platform worked well near the landfill, but data were not as clear from the dairy farm because the cattle were indoors. The team expects a stronger source signature when livestock are outdoors, however.
Instrument Comparisons
In addition to the field and mobile surveys, Dr. Lowry and his team were interested in assessing measurements of known standards. At the National Physical Laboratory in nearby Teddington, West London, they measured a gas standard that is depleted of 13C, with a known value of -41.9‰. The LI-7825 offsets were <0.1‰ following a three-point calibration, which is “as good as can be expected,” according to Lowry.
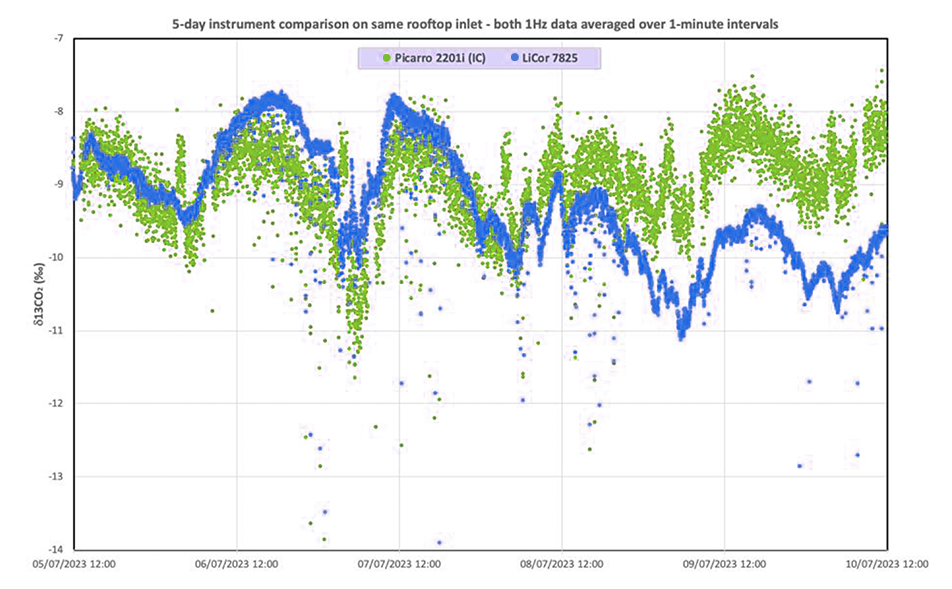
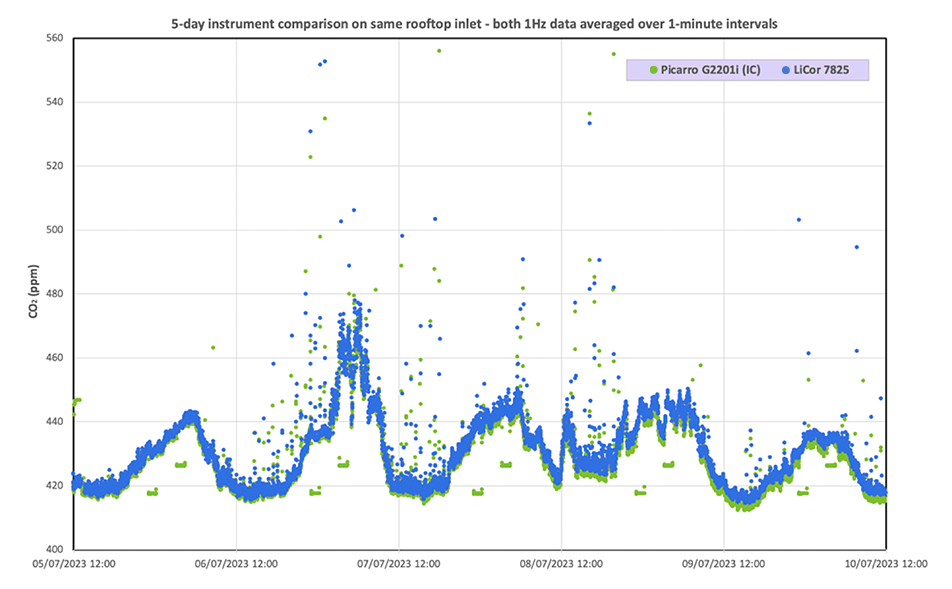
Having measured known standards, the team moved on to instrument comparisons, in which they compared measurements from the LI-7825 with those from a Picarro G2201i gas analyzer. Sampling was from a roof-mounted air intake at the Imperial College, London. Data showed periodic spikes of higher-than-average CO2 concentrations associated with 13C depletion. Periodic 30-second spikes occurred at intervals of 42 to 43 minutes when the wind originated from the south-southeast to south-southwest. After some deduction, Dr. Lowry determined that the data indicate emissions from a nearby boiler, which is being activated on regular intervals.
View Data
After his evaluation, Dr. Lowry points out that the instrument performed as well as the stated specification for δ13C measurements. With regular calibration checks and zero adjustments, the instrument reports isotopic measurements with less noise than the comparison instrument.
The LI-7825 is designed to make CO2 isotope measurements more widespread by combining low operating costs, high-precision measurements, and portability. With more widespread measurements of isotopes of carbon in CO2, sources of CO2 can be more easily identified. Source attribution is difficult but is easier with instruments like the LI-7825, and this information can help humans make better decisions about climate and CO2 emissions.
Dr. Lowry is a stable-isotope geochemist at Royal Holloway, University of London (RHUL) in England.
Contact LI-COR to learn more about the LI-7825 CO2 Isotope/NH3 Trace Gas Analyzer.